Sustainable Potassium Sorbate Production from Triacetic Acid Lactone in Food-Grade Solvents
Themes: Conversion, Sustainability
Keywords: Bioproducts, Catalysis
Citation
Kim, M.S., Bhagwat, S., Santiago-Martínez, L., Shi, X., Choi, K., Guest, J.S., Huber, G.W. April 29, 2025. Data from: “Sustainable Potassium Sorbate Production from Triacetic Acid Lactone in Food-grade Solvents.” University of Illinois Urbana-Champaign. DOI: 10.13012/B2IDB-0247002_V1.
Overview
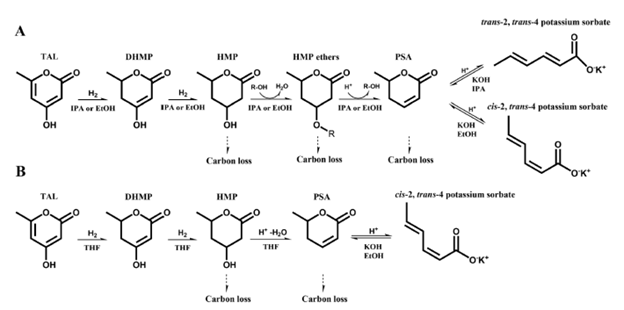
This study advances the production of potassium sorbate (KS) from triacetic acid lactone (TAL) utilizing food-grade solvents, ethanol (EtOH) and isopropyl alcohol (IPA). We have previously demonstrated the route to produce KS from TAL in tetrahydrofuran (THF) as the main solvent, but the use of THF is associated with environmental and health risks especially for food applications. The process employs a catalytic approach in food-grade solvents and includes three main steps: hydrogenation, etherification and hydrolysis, and ring-opening hydrolysis to produce KS from TAL. In the synthesis of KS from TAL, the use of IPA leads to higher yields and reduced reaction times compared to EtOH. As a result, the overall reaction time in IPA was reduced to 35.7 h, compared to 42.1 h in our previous study using THF and EtOH, while achieving a comparable KS yield of 84% from TAL. The synthesized KS exhibits a trans-2, trans-4 geometrical configuration, identical to that of commercially available KS. Through techno-economic analysis (TEA) and life cycle assessment (LCA), we estimated full-scale production of KS from sugarcane with the developed process in IPA could achieve a minimum product selling price (MPSP) of $8.27 per kg with a range of $7.06–10.16 per kg [5th–95th percentiles from 6000 Monte Carlo simulations] and a carbon intensity (CI) of 13.7 [9.6–18.6] kg CO2-eq per kg. This study highlights the synthesis of KS from TAL using food-grade solvents, demonstrating improved economic viability and environmental sustainability compared to our previous research (MPSP of $9.68 per kg [$8.47–11.45 per kg] and CI of 16.2 [12.0–21.2] kg CO2-eq per kg), as the total required reaction decreases while achieving the comparable overall yield of KS from TAL.
Data
Illinois Data Bank includes:
- Catalytic performance
- TAL hydrogenation
- HMP conversion
- Analyses parameters