Photoenzymatic Stereoablative Enantioconvergence of γ-chiral Oximes via Hydrogen Atom Transfer
Themes: Conversion
Keywords: Bioproducts, Catalysis
Citation
Zhang, Z., Li, M., Harrison, W., Lu, J., Zhao, Z., Yuan, Y., Zhao, H. June 24, 2025. “Photoenzymatic Stereoablative Enantioconvergence of γ-Chiral Oximes via Hydrogen Atom Transfer.” Nature Catalysis. DOI: 10.1038/s41929-025-01347-0.
Overview
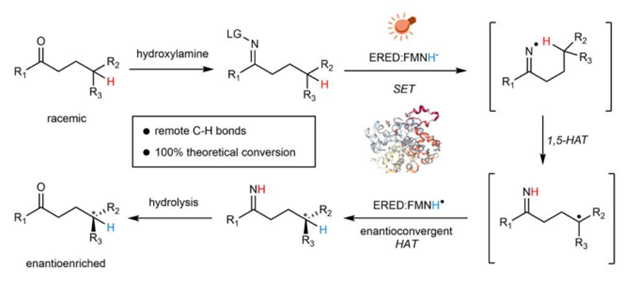
Producing enantioenriched molecules from racemic mixtures is essential for manufacturing. Traditional methods such as resolution, deracemization and enantioconvergent catalysis primarily involve separating or converting enantiomers without altering their structures, or functionalization of stereocentres at or proximal to functional groups. However, there are challenges in enantioselectively forging C–H bonds that are remote from functional groups via hydrogen atom transfer (HAT) with these methods. Here we introduce a strategy for the photoenzymatic stereoablative enantioconvergence of γ-chiral oximes using repurposed flavin-dependent ene-reductases. A photoinduced single-electron reduction of the γ-chiral oxime by an ene-reductase generates an iminyl radical, which then undergoes stereoablative 1,5-HAT at the γ-stereocentre. Subsequent chiral reconstruction through enzymatic HAT and spontaneous imine hydrolysis yields the γ-chiral ketone with high enantioselectivity. This work provides a robust method for remote stereoablative enantioconvergent HAT and broadens the synthetic utility of photobiocatalysis.
Data
Download (1.5 MB) includes:
- Photoenzymatic enantioconvergence data
- Control experiments
- Condition optimizations
- Supplemental data