Photoenzymatic Enantioselective Intermolecular Radical Hydroalkylation: Experimental Crystal Structure Determination
Themes: Conversion
Keywords: Catalysis
Citation
Huang, X., Wang, B., Wang, Y., Jiang, G., Feng, J., Zhao, H. Dec. 30, 2019. CCDC 1989831: Experimental Crystal Structure Determination, DOI: 10.5517/ccdc.csd.cc24sl3n
Overview
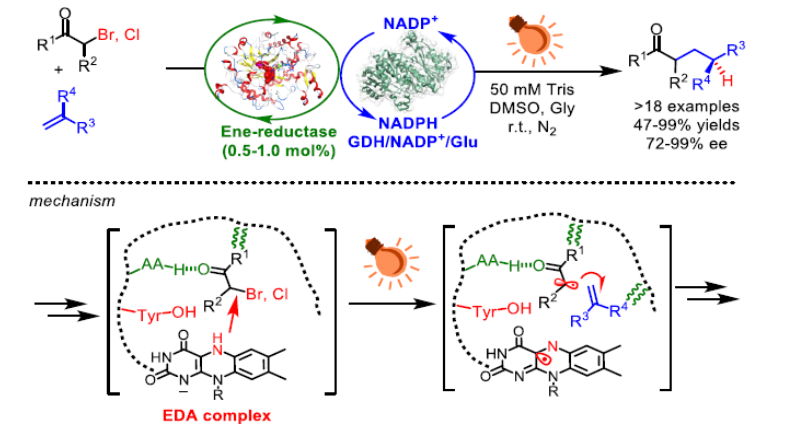
Enzymes are increasingly explored for use in asymmetric synthesis, but their applications are generally limited by the reactions available to naturally occurring enzymes. Recently, interest in photocatalysis has spurred the discovery of novel reactivity from known enzymes. However, so far photoinduced enzymatic catalysis has not been used for the cross-coupling of two molecules. For example, the intermolecular coupling of alkenes with α-halo carbonyl compounds through a visible-light-induced radical hydroalkylation, which could provide access to important γ-chiral carbonyl compounds, has not yet been achieved by enzymes. The major challenges are the inherent poor photoreactivity of enzymes and the difficulty in achieving stereochemical control of the remote prochiral radical intermediate. Here we report a visible-light-induced intermolecular radical hydroalkylation of terminal alkenes that does not occur naturally, catalysed by an ‘ene’ reductase using readily available α-halo carbonyl compounds as reactants. This method provides an efficient approach to the synthesis of various carbonyl compounds bearing a γ-stereocentre with excellent yields and enantioselectivities (up to 99 percent yield with 99 percent enantiomeric excess), which otherwise are difficult to access using chemocatalysis. Mechanistic studies suggest that the formation of the complex of the substrates (α-halo carbonyl compounds) and the ‘ene’ reductase triggers the enantioselective photoinduced radical reaction. Our work further expands the reactivity repertoire of biocatalytic, synthetically useful asymmetric transformations by the merger of photocatalysis and enzyme catalysis.
Data
Additional download (19MB) includes:
- Visible-light-induced enantioselective intermolecular radical hydroalkylation catalyzed by ‘ene’ reductases reaction development
- Ultraviolet-visible absorption spectra indicating the formation of the EDA complex
- Crystal data and structure refinement for the crystal 3f
- Bond lengths and angles for the crystal 3f