Metabolic Engineering of Rhodotorula toruloides IFO0880 Improves C16 and C18 Fatty Alcohol Production from Synthetic Media
Themes: Conversion
Keywords: Genome Engineering, Genomics
Citation
Schultz, J.C., Mishra, S., Gaither, E., Mejia, A., Dinh, H., Maranas, C., Zhao, H. Feb. 19, 2022. “Metabolic Engineering of Rhodotorula toruloides IFO0880 Improves C16 and C18 Fatty Alcohol Production from Synthetic Media.” Microbial Cell Factories 21:26. DOI: 10.1186/s12934-022-01750-3.
Overview
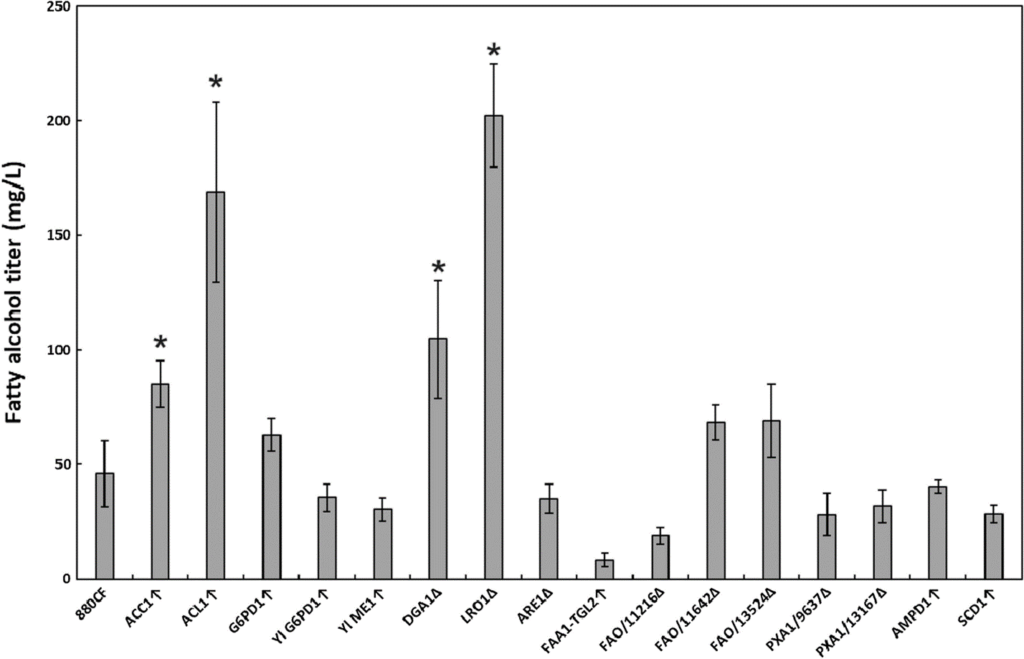
The oleaginous, carotenogenic yeast Rhodotorula toruloides has been increasingly explored as a platform organism for the production of terpenoids and fatty acid derivatives. Fatty alcohols, a fatty acid derivative widely used in the production of detergents and surfactants, can be produced microbially with the expression of a heterologous fatty acyl-CoA reductase. Due to its high lipid production, R. toruloides has high potential for fatty alcohol production, and in this study several metabolic engineering approaches were investigated to improve the titer of this product. Fatty acyl-CoA reductase from Marinobacter aqueolei was co-expressed with SpCas9 in R. toruloides IFO0880 and a panel of gene overexpressions and Cas9-mediated gene deletions were explored to increase the fatty alcohol production. Two overexpression targets (ACL1 and ACC1, improving cytosolic acetyl-CoA and malonyl-CoA production, respectively) and two deletion targets (the acyltransferases DGA1 and LRO1) resulted in significant (1.8 to 4.4-fold) increases to the fatty alcohol titer in culture tubes. Combinatorial exploration of these modifications in bioreactor fermentation culminated in a 3.7 g/L fatty alcohol titer in the LRO1Δ mutant. As LRO1 deletion was not found to be beneficial for fatty alcohol production in other yeasts, a lipidomic comparison of the DGA1 and LRO1 knockout mutants was performed, finding that DGA1 is the primary acyltransferase responsible for triacylglyceride production in R. toruloides, while LRO1 disruption simultaneously improved fatty alcohol production, increased diacylglyceride and triacylglyceride production, and increased glucose consumption. The fatty alcohol titer of fatty acyl-CoA reductase-expressing R. toruloides was significantly improved through the deletion of LRO1, or the deletion of DGA1 combined with overexpression of ACC1 and ACL1. Disruption of LRO1 surprisingly increased both lipid and fatty alcohol production, creating a possible avenue for future study of the lipid metabolism of this yeast.
Data
- Fatty alcohol titers
- Strains, Plasmids and Primers
- Disruption efficiency