Metabolic Engineering of β-Oxidation to Leverage Thioesterases for Production of 2-Heptanone, 2-Nonanone, and 2-Undecanone
Themes: Conversion
Keywords: Metabolomics
Citation
Yan, Q., Simmons, T.R., Cordell, W.T., Hernandez Lozada, N.J., Breckner, C.J., Chen, X., Jindra, M.A., Pfleger, B.F. May 29, 2020. “Metabolic Engineering of β-Oxidation to Leverage Thioesterases for Production of 2-Heptanone, 2-Nonanone, and 2-Undecanone.” Metabolic Engineering. DOI: 10.1016/j.ymben.2020.05.008.
Overview
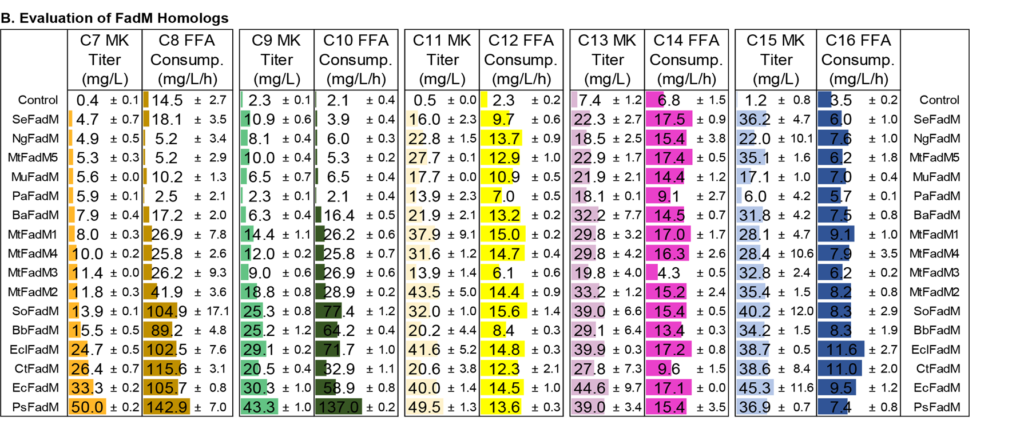
Medium-chain length methyl ketones are potential blending fuels due to their cetane numbers and low melting temperatures. Biomanufacturing offers the potential to produce these molecules from renewable resources such as lignocellulosic biomass. In this work, we designed and tested metabolic pathways in Escherichia coli to specifically produce 2-heptanone, 2-nonanone and 2-undecanone. We achieved substantial production of each ketone by introducing chain-length specific acyl-ACP thioesterases, blocking the β-oxidation cycle at an advantageous reaction, and introducing active β-ketoacyl-CoA thioesterases. Using a bioprospecting approach, we identified 15 homologs of E. coli β-ketoacyl-CoA thioesterase (FadM) and evaluated the in vivo activity of each against various chain length substrates. The FadM variant from Providencia sneebia produced the most 2-heptanone, 2-nonanone, and 2-undecanone, suggesting it has the highest activity on the corresponding β-ketoacyl-CoA substrates. We tested enzyme variants, including acyl-CoA oxidases, thiolases, and bi-functional 3-hydroxyacyl-CoA dehydratases to maximize conversion of fatty acids to β-keto acyl-CoAs for 2-heptanone, 2-nonanone, and 2-undecanone production. In order to address the issue of product loss during fermentation, we applied a 20% (v/v) dodecane layer in the bioreactor and built an external water cooling condenser connecting to the bioreactor heat-transferring condenser coupling to the condenser. Using these modifications, we were able to generate up to 4.4 g/L total medium-chain length methyl ketones.
Data
- FadM Activity
- Plasmid expression
- Time courses of Methyl Ketone, OD600 and Glycerol in reactions
- Plasmids and strains used
- Reaction Rates
- Protein sequences