Evaluation of Strategies to Narrow the Product Chain-Length Distribution of Microbially Synthesized Free Fatty Acids
Themes: Conversion
Keywords: Genomics
Citation
Jindra, M.A., Choe, K., Chowdhury, R., Kong, R., Ghaffari, S., Sweedler, J.V., Pfleger, B.F. March 1, 2023. “Evaluation of Strategies to Narrow the Product Chain-Length Distribution of Microbially Synthesized Free Fatty Acids.” Metabolic Engineering. DOI: 10.1016/j.ymben.2023.02.012.
Overview
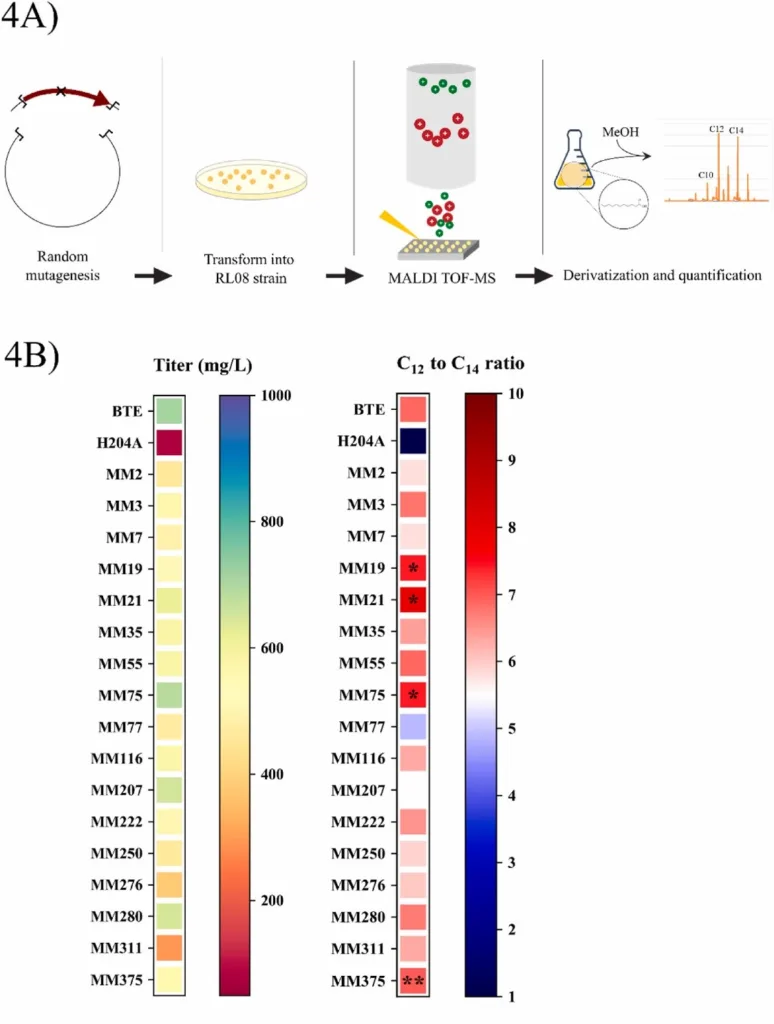
The dominant strategy for tailoring the chain-length distribution of free fatty acids (FFA) synthesized by heterologous hosts is expression of a selective acyl-acyl carrier protein (ACP) thioesterase. However, few of these enzymes can generate a precise (greater than 90% of a desired chain-length) product distribution when expressed in a microbial or plant host. The presence of alternative chain-lengths can complicate purification in situations where blends of fatty acids are not desired. We report the assessment of several strategies for improving the dodecanoyl-ACP thioesterase from the California bay laurel to exhibit more selective production of medium-chain free fatty acids to near exclusivity. We demonstrated that matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-ToF MS) was an effective library screening technique for identification of thioesterase variants with favorable shifts in chain-length specificity. This strategy proved to be a more effective screening technique than several rational approaches discussed herein. With this data, we isolated four thioesterase variants which exhibited a more selective FFA distribution over wildtype when expressed in the fatty acid accumulating E. coli strain, RL08. We then combined mutations from the MALDI isolates to generate BTE-MMD19, a thioesterase variant capable of producing free fatty acids consisting of 90% of C12 products. Of the four mutations which conferred a specificity shift, we noted that three affected the shape of the binding pocket, while one occurred on the positively charged acyl carrier protein landing pad. Finally, we fused the maltose binding protein (MBP) from E. coli to the N – terminus of BTE-MMD19 to improve enzyme solubility and achieve a titer of 1.9 g per L of twelve-carbon fatty acids in a shake flask.
Data
- Strains and Variants
- Parameters
- Vector Summaries