Catalytic Strategy for Conversion of Triacetic Acid Lactone to Potassium Sorbate
Themes: Conversion
Keywords: Catalysis, Modeling
Citation
Kim, M.S., Choi, D., Ha, J., Choi, K., Yu, J.H., Dumesic, J.A., Huber, G.W. Oct. 18, 2023. “Catalytic Strategy for Conversion of Triacetic Acid Lactone to Potassium Sorbate.” ACS Catalysis. DOI: 10.1021/acscatal.3c02775.
Overview
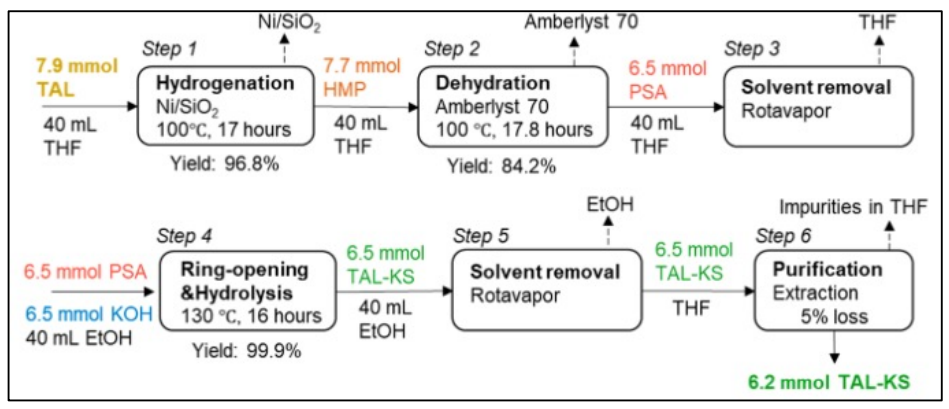
This study shows a new route to produce potassium sorbate (KS) from triacetic acid lactone (TAL), which is a chemical platform that can be biologically synthesized from natural sources. Sorbic acid and its potassium salt (KS) are widely used as preservatives in foods and pharmaceuticals. Three steps are used to produce KS from TAL: 1) hydrogenation of TAL into 4-hydroxy-6-methyltetrahydro-2-pyrone (HMP), 2) dehydration of HMP to parasorbic acid (PSA), and 3) ring-opening and hydrolysis of PSA to KS. TAL can be fully hydrogenated over Ni/SiO2 to give near quantitative yields of HMP. A three-step reaction kinetics model was developed for dehydration of HMP into PSA. This model was used to show that the highest PSA yield occurs at low temperatures. An experimental PSA yield of 84.2% with respect to TAL was obtained which agreed with the prediction of the reaction kinetics model. KOH was used as a coreactant for the ring-opening hydrolysis of PSA to produce >99.9% yield of KS from PSA. Tetrahydrofuran (THF) was used to purify the TAL derived-KS (TAL-KS). The TAL-KS had a KS purity of 95.5%. The overall yield of TAL-KS with respect to TAL was calculated to be 77.3%. TAL-KS produced in this study had similar antimicrobial activities as commercial KS.
Data
Download (345.KB) includes:
- HMP dehydration at 100, 120, and 140 degrees C
- PSA yield at 100, 120, and 140 degrees C
- PSA to KS conversion