A Designed Heme-[4Fe-4S] Metalloenzyme Catalyzes Sulfite Reduction Like the Native Enzyme
Themes: Conversion
Keywords: Catalysis
Citation
Mirts, E.N., Petrik, I.D., Hosseinzadeh, P., Nilges, M.J., Lu, Y. Sept. 14, 2018. “Data from A Designed Heme-[4Fe-4S] Metalloenzyme Catalyzes Sulfite Reduction Like the Native Enzyme.” University of Illinois Urbana-Champaign. DOI: 10.13012/B2IDB-7649944_V1.
Overview
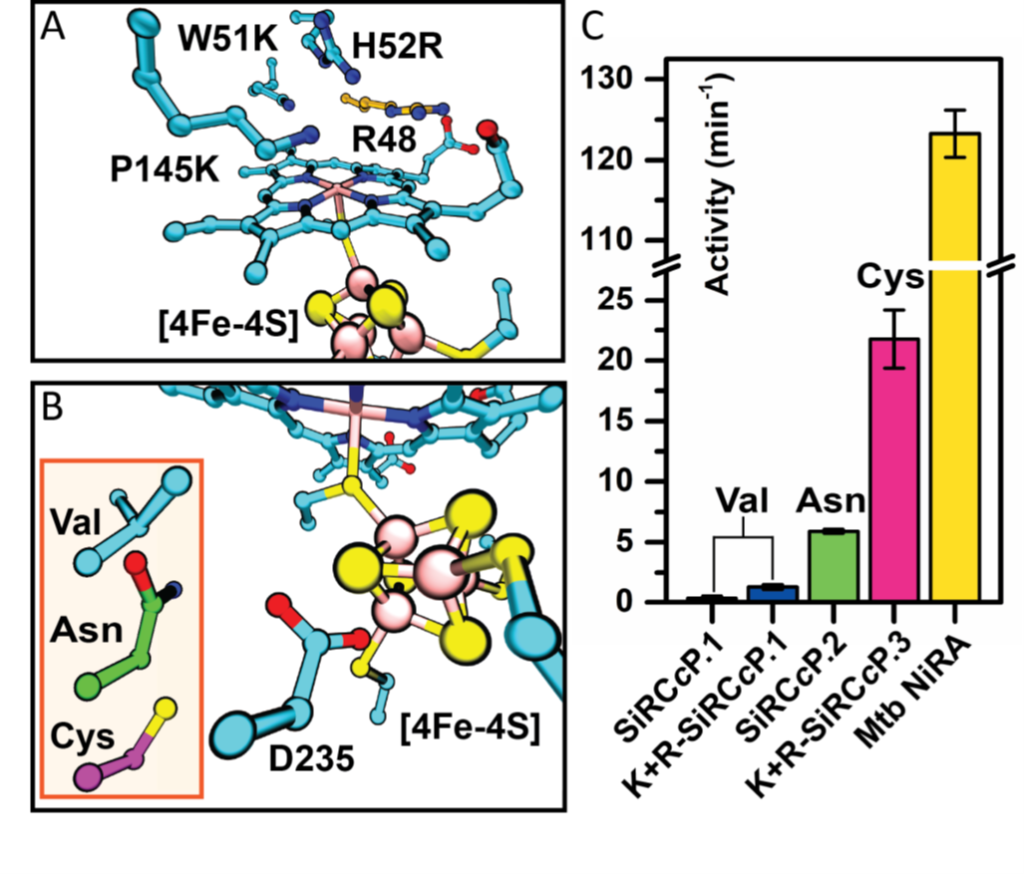
Enzymatic reduction of oxyanions such as sulfite (SO32−) requires the delivery of multiple electrons and protons, a feat accomplished by cofactors tailored for catalysis and electron transport. Replicating this strategy in protein scaffolds may expand the range of enzymes that can be designed de novo. Mirts et al. selected a scaffold protein containing a natural heme cofactor and then engineered a cavity suitable for binding a second cofactor—an iron-sulfur cluster (see the Perspective by Lancaster). The resulting designed enzyme was optimized through rational mutation into a catalyst with spectral characteristics and activity similar to that of natural sulfite reductases.
Data
Illinois Data Bank includes:
- EXAFS parameters
- Sulfite reductase SiRCcP Activities