Photoenzymatic Asymmetric Hydroamination for Chiral Alkyl Amine Synthesis
Themes: Conversion
Keywords: Bioproducts, Catalysis
Citation
Harrison, W., Jiang, G., Zhang, Z., Li, M., Chen, H., Zhao, H. April 5, 2024. “Data for Photoenzymatic Asymmetric Hydroamination for Chiral Alkyl Amine Synthesis.” University of Illinois Urbana-Champaign. DOI: 10.13012/B2IDB-7913244_V1.
Overview
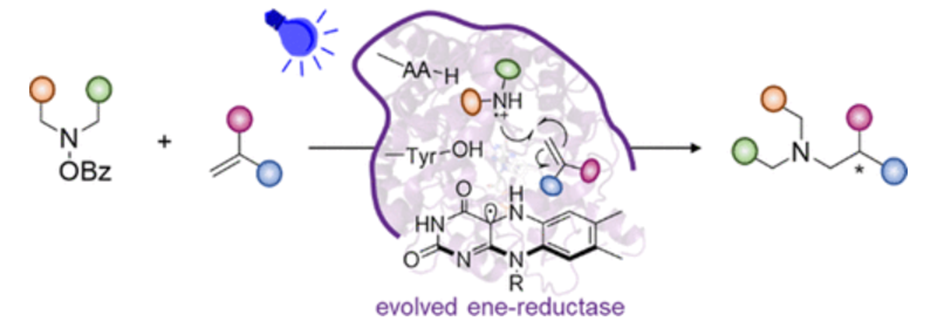
Chiral alkyl amines are common structural motifs in pharmaceuticals, natural products, synthetic intermediates, and bioactive molecules. An attractive method to prepare these molecules is the asymmetric radical hydroamination; however, this approach has not been explored with dialkyl amine-derived nitrogen-centered radicals since designing a catalytic system to generate the aminium radical cation, to suppress deleterious side reactions such as α-deprotonation and H atom abstraction, and to facilitate enantioselective hydrogen atom transfer is a formidable task. Herein, we describe the application of photoenzymatic catalysis to generate and harness the aminium radical cation for asymmetric intermolecular hydroamination. In this reaction, the flavin-dependent ene-reductase photocatalytically generates the aminium radical cation from the corresponding hydroxylamine and catalyzes the asymmetric intermolecular hydroamination to furnish the enantioenriched tertiary amine, whereby enantioinduction occurs through enzyme-mediated hydrogen atom transfer. This work highlights the use of photoenzymatic catalysis to generate and control highly reactive radical intermediates for asymmetric synthesis, addressing a long-standing challenge in chemical synthesis.
Data
- Study primers
- Screening data
- Reaction images