Photoinduced Chemomimetic Biocatalysis for Enantioselective Intermolecular Radical Conjugate Addition
Themes: Conversion
Keywords: Catalysis
Citation
Huang, X., Feng, J., Cui, J., Jiang, G., Harrison, W., Zang, X., Zhou, J., Wang, B., Zhao, H. May 2, 2022. “Photoinduced Chemomimetic Biocatalysis for Enantioselective Intermolecular Radical Conjugate Addition.” Nature Catalysis. DOI: 10.1038/s41929-022-00777-4.
Overview
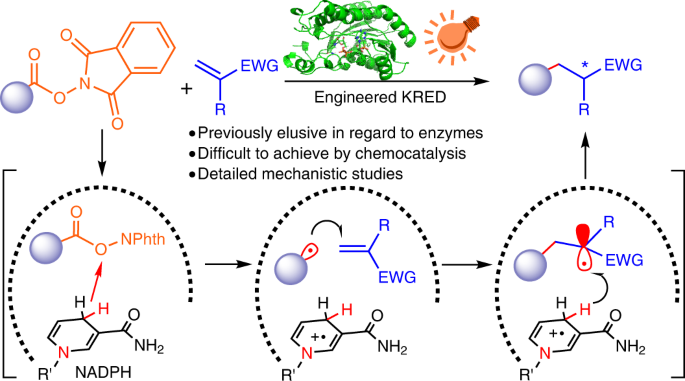
Exploiting nature’s catalysts for non-natural transformations that are inaccessible to chemocatalysis is highly desirable but challenging. On the one hand, the widespread nicotinamide-dependent oxidoreductases have not been utilized for single-electron-transfer-induced bimolecular cross-couplings; on the other, the addition of catalytic asymmetric radical conjugate to terminal alkenes remains a challenge owing to strong racemic background reaction and unselective termination of prochiral radical species. Here we report a chemomimetic biocatalysitic approach for construction of alpha-carbonyl stereocentres via an unnatural intermolecular conjugate addition of N-(acyloxy)phthalimides-derived radicals with acceptor-substituted terminal alkenes, by combination of visible-light excitation and nicotinamide-dependent ketoreductases (KREDs). Based on protein crystal structure, we engineered KREDs via a semi-rational mutagenesis strategy to improve reaction outcomes with a small and high-quality variants library. Mechanistic investigations combining wet experiments, crystallographic studies and computational simulations demonstrate that the repurposed biocatalyst can suppress racemic background reaction and unselected side reactions, yielding enantioselectivity that is challenging to achieve by chemocatalysis.
Data
Atomic coordinates:
Configurations of molecular dynamics simulations
Illinois Data Bank includes:
- Primers
- Interaction Energies
- Radicals